Salt Facts
Where does the salt come from?
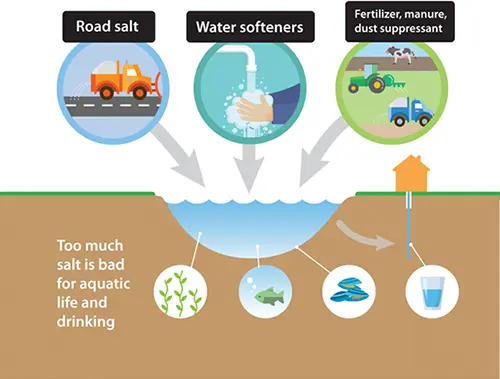
Winter Road Salt
- Sodium Chloride (NaCl), also known as table salt or rock salt when spread on the road, is the most commonly used substance to deice roads and highways.
- Deicers containing chloride are widely used to remove snow and ice from paved surfaces such as roads, parking lots, driveways, and sidewalks during winter. Chloride lowers the freezing point of water, making it easier to remove snow and ice from these surfaces.
- Although rock salt is cheap and effective at melting snow and ice, its use may cause damage to infrastructure, property, and the environment.
- Salt was first used for deicing roads in the 1930s but only became widespread in the 1960s after winter maintenance personnel discovered its effectiveness when used in conjunction with plowing.
Water Softeners
- People with hard water use water softeners to remove excess nutrients. However, water softeners add chlorides to the water, ending in our waterways.
Fertilizer, Manure, and Dust Suppressant
- Potassium chloride is a common fertilizer that can benefit plants and increase plant yields. However, if not applied appropriately, it washes off into the waterways.
- Chloride can leach from livestock and poultry manure on the rural landscape runoff.
- Calcium chloride is the salt applied to gravel roads during warmer months to suppress dust on gravel roads by trapping moisture. known as rock salt when spread on the road because of its much larger granules.

Why is Road Salt Used?
Chloride deicers are extensively used in Boone County to ensure public safety on transportation surfaces. Road salt, which is essentially sodium chloride, is used to lower the freezing point of water. Even when the temperature is below the normal freezing point of water, which is 32 degrees, salt can effectively melt ice.
The Cost and Impact of Road Salt
Besides the cost of materials and labor required to salt streets in the winter, there are other costs to winter salt usage.
Infrastructure Damage
Rock salt is widely used as a de-icing agent on roads and bridges during winter. However, it has corrosive properties that can cause damage to cars, trucks, bridges, and roads. The Environmental Protection Agency reports that the cost of annual repairs due to rock salt damage in the US alone is approximately 5 billion dollars.
Even after the snow and ice have melted, the salt residue remains on the roads and can corrode the undercarriage of cars and the embedded rebar in roads and bridges. The salts can also cause damage to residential plumbing and cause lead and other harmful minerals to leach from public water distribution lines. Additionally, runoff from piles of rock salt remaining on the road after storms can harm nearby waterways.
Water(Environmental) Impacts
Salt does not break down or degrade. Once it is in the water or soil, removing it is challenging and expensive. Salt in the environment can have ripple effects, so it's not just salt that is harming plants and animals. The salt can also mobilize other contaminants.
The only way to reduce the impact of salt on the environment is to reduce the amount of salt we use.
Fresh Waterways

After snowstorms, the levels of salt in waterways tend to increase, especially in highly urbanized areas. This happens because melting snow carries road salt into storm drains, which then dumps into creeks and rivers. Water that contains salt has a higher density than water that does not, causing it to sink to the bottom of the water body. This can lead to chemical stratification and disrupt the lake mixing patterns, as stated by the (New Hampshire Department of Environmental Service, N.D.; Novotny et al., 2007).
Animal Health

Road salt can have detrimental effects on wildlife as it contaminates waterways, reduces the populations of organisms, and impacts food sources. When salt is applied to roads and sidewalks, it can be carried by runoff into nearby streams, rivers, ponds, and lakes. Elevated levels of salt in the water that result from this practice can be toxic to plants and animals that rely on these water sources. For instance, amphibians exposed to increased salinity while in the egg have a high frequency of malformations when hatched.
Drinking Water
If road salt is not used responsibly, the salty water can end up in our drinking water because private wells and public water utilities draw water from local streams, reservoirs, and aquifers.
Road salt can soak into our groundwater, which can directly introduce excess sodium into drinking water and pose a health risk, especially to people on low-sodium diets. Salt can also leach chemicals from pipes, contaminating drinking water.
Plants
Roadside vegetation can be negatively impacted by chloride absorption through the plant roots or from accumulating on the foliage and branches. Road salt runoff can also cause soil salinization, the accumulation of salt in soil that affects the environment's health. Salt absorbs water, resulting in less water being available to plants. That, in turn, causes the drying out (or desiccation) and eventual death of many plants. Reduced plant populations can have cascading effects on local ecosystems. For example, if one tree species becomes less abundant due to road salt, the organisms that depend on that tree for food or shelter may also suffer.
The symptoms associated with salt impacts are similar to those of a drought: stunted growth, brown and falling leaves/needles, dying limbs, and premature plant depths (National Research Council, 1991).
Pets
Salt crystals and salty slush can adhere to the pet's feet and cause irritation, soreness, and burning of the pads or skin. Salt can also be ingested when pets lick their paws or drink water contaminated with salt outside. Excessive ingestion can lead to vomiting, diarrhea, unnaturally intense thirst, or, in extreme cases, poisoning.